"The woman is created for a man, not a man for a woman" - such a postulate ...


Law radioactive decay -- physical Lawdescribing the dependence of the intensity of the radioactive decay on the time and number of radioactive atoms in the sample. Opened Frederick Soddy and Ernest Rutherford, each of which was later awarded Nobel Prize. They found him experimentally and published in 1903 in the works "Comparative study of radium and thorium radioactivity" and "radioactive transformation", formulating as follows:
"In all cases, when one of the radioactive products was separated and its activity was investigated independently of the radioactivity of the substance from which it was formed, it was found that activity with all studies decreases with time under the law of geometric progression."
With the help of the Bernoulli Theorem, the following conclusion was obtained: the speed of transformation is proportional to the number of systems that have not yet been transformed.
There are several formulations of the law, for example, in the form of a differential equation:
radioactive decay atom Quantum Mechanical
which means that the number of decays? DN, which occurred in the short time interval of DT, in proportion to the number of N atoms in the sample.
In the above mathematical expression - a constant decay, which characterizes the likelihood of radioactive decay per unit of time and having dimension s? 1. The minus sign indicates the decline in the number of radioactive nuclei with time.
The solution of this differential equation has the form:
where - the initial number of atoms, that is, the number of atoms for
Thus, the number of radioactive atoms is reduced over time according to the exponential law. The rate of decay, that is, the number of decays per unit of time also falls exponentially.
Differentiating expression for the dependence of the number of atoms from time to time, we obtain:
where - the rate of decay at the initial moment of time
Thus, the dependence on the time of the number of unsuccessful radioactive atoms and the decay velocity is described by the same constant
In addition to the decay constant, the radioactive decay characterizes two more derivatives from it by constants:
1. average lifetime
The lifetime of the quantum mechanical system (particles, kernels, atoms, energy level, etc.) - a period of time during which the system decays with a probability where E \u003d 2,71828 ... - the number of Euler. If independent particle ensemble is considered, then during the time the number of remaining particles decreases (on average) in rely on the number of particles at the initial moment. The concept of "lifetime" applies under conditions when an exponential decay occurs (that is, the expected number of surviving particles n depends on time t as
where N 0 is the number of particles at the initial moment). For example, for neutrino oscillations, this term cannot be applied.
Living lifetime is associated with a half-life T 1/2 (time during which the number of surviving particles on average is halucing the following ratio:
The value, the inverse lifetime, is called a constant decay:
The exponential decay is observed not only for quantum mechanical systems, but also in all cases when the probability of an irreversible transition of the system element to another state per unit of time does not depend on time. Therefore, the term "lifetime" is used in areas that are quite distinguished from physics, for example, in the theory of reliability, pharmacology, chemistry, etc. The processes of this kind are described by a linear differential equation.
meaning that the number of elements in the initial state decreases with a speed proportional N (T) /. The proportionality coefficient is so, in pharmacokinetics after one-time introduction chemical compound The compound is gradually destroyed in biochemical processes and is derived from the body, and if it does not cause significant changes in the rate of biochemical processes acting on it (i.e., the impact linearly) is described by the exponential law, and we can talk about The lifetime of the chemical compound in the body (as well as the period of half-life and the decay constant).
2. The half-life period
The half-life of the quantum mechanical system (particles, nuclei, atom, energy level, etc.) - Time T *, during which the system decays with a probability of 1/2. If independent particle ensemble is considered, then for one half-life, the number of surviving particles will decrease by an average of 2 times. The term applies only to exponentially disintegrating systems.
It should not be assumed that all particles taken at the initial moment will disintegrate in the two periods of the half-life. Since each half-life decreases the number of surviving particles twice, during 2T ѕ, a quarter remains from the initial number of particles, for 3T ѕ - one eighth, etc. In general, the share of surviving particles (or, more precisely, the probability of survival P for this particle) Depends on time t as follows:
The half-life, average lifetime and constant decay are associated with the following relations obtained from the law of radioactive decay:
Since the half-life period is about 30.7% shorter than the average lifetime.
In practice, the half-life is determined by measuring the activity of the studied drug at certain intervals. Considering that the activity of the drug is proportional to the number of atoms of the disintegrating agent, and using the law of radioactive decay, the half-life of this substance can be calculated
Partial half of the half-life
If the system with a half-life T 1/2 may decay over several channels, for each of them you can determine the partial period of the half-life. Let the probability of decay according to the i-MU channel (branching coefficient) equal to P i. Then the partial period of the half-life according to the i-MU channel is equal
Partial it makes sense of a half-life, which would have this system if "turn off" all decay channels except the i-th. Since by definition, for any breakdown channel.
Stability of the half-life period
In all those observed cases (except for some isotopes dislike Electronic capture) The half-life period was permanent (separate reports on the change in the period were caused by the lack of experiment accuracy, in particular, incomplete purification from highly active isotopes). In connection with this, the half-life is considered unchanged. This basis is based on the determination of absolute geological age mountain breeds, and also varied coupler method of determining the age of biological remains.
The assumption of the changeability of the half-life is used by creationists, as well as representatives of the T.N. " alternative science»To refute the scientific dating of rocks, residues of living beings and historical finds, in order to further refute the scientific theories built using such dating. (See, for example, Articles Creationism, Scientific Creationism, Criticism of Evolutionism, Turin Cloak).
The variability of a constant decay for an electron capture was observed in the experiment, but it lies within a percentage throughout the pressure and temperature range available in the laboratory. The half-life in this case varies in connection with some (rather weak) dependence of the density of the wave function of orbital electrons in the vicinity of the nucleus from pressure and temperature. Significant changes in the constant decay were also observed for strongly ionized atoms (so, in the maximum case of a completely ionized nucleus, an electron capture can occur only when the kernel interacts with the free plasma electrons; in addition, the decay, permitted for neutral atoms, in some cases for highly ionized atoms can Be prohibited kinematically). All these options for changing constant decay are obviously cannot be brought to "refutation" of radio chronological datches, since the error of the radioichronometric method itself for most isotopes-chronometers is more than a percent, and high-incredited atoms in natural objects On earth can not exist any long time.
The search for possible variations of the periods of the half-life of radioactive isotopes, both at the present time and for billions of years, is interesting due to the hypothesis of ovariations of the values \u200b\u200bof fundamental constants in physics (constant fine structure, Fermi constants, etc.). However, thorough measurements have not yet brought the result - within the error of the experiment, the change in the half-life were not found. Thus, it was shown that for 4.6 billion years of the B-decay constant of Samaria-147 has changed no more than 0.75%, and for B-decay of Rhenium-187, the change during the same time does not exceed 0.5%; In both cases, the results are compatible with the lack of such changes at all.
Changing the number of radioactive cores over time. Rutherford and Soddy in 1911, summarizing experimental results, showed that atoms of some elements experience successive transformations, forming radioactive families, where each member arises from the previous one and, in turn, forms the subsequent.
This is conveniently illustrated by the example of radon formation from radium. If you put a gas analysis in a sealed ampoule, a few days will show that helium and radon appears in it. Helium is stable, and therefore it accumulates, Radon itself decays. Curve 1 in fig. 29 characterizes the law of the decay of radon in the absence of radium. At the same time, the ratio of the number of unprecedented radon nuclei to their initial number is seen on the ordinate axis. Curve 2 shows how the number of radioactive radon nuclei changes in the presence of radium.
Experiments carried out with radioactive substances showed that no external conditions (Heating to high temperatures
magnetic and electric fields, large pressure) cannot affect the character and rate of decay.
Radioactivity is the property of the atomic nucleus and for this type nuclei in a certain energy conditionThe probability of radioactive decay per unit of time is constant.

Fig. 29. Dependence of the number of active radon nuclei from time
Since the decay process is spontaneous (spontaneous), then the change in the number of nuclei due to decay during the period of time is determined only by the number of radioactive cores at the moment and in proportion to the time intermediate
![]()
where constant characterizing the rate of decay. Integrating (37) and consider what we get
![]()
i.e. the number of cores decreases under the exponential law.
This law refers to statistical averages and is valid only with enough big number Particles. The value of X is called a constant radioactive decay, has a dimension and characterizes the likelihood of the decay of one atom in one second.
For the characteristics of radioactive elements, the concept of a half-life is also introduced under it. Time is understood during which half of the cash number of atoms decomposes. Substituting the condition to equation (38), we get
![]()
from where, logarithming, we find that
and half-life
![]()
With the exponential law of radioactive decay at any time there is an excellent probability from zero to find not yet broken kernels. The lifetime of these cores exceeds
On the contrary, other kernels that have stayed by this time lived different time, less average lifetime for this radioactive isotope is defined as

Recognition we get

Consequently, the average life time of the radioactive nucleus is equal to the inverse of the constant decay of J. During the initial number of nuclei decreases at times.
For processing experimental results, it is convenient to present equation (38) in another form:
![]()
The value is called the activity of this radioactive preparation, it determines the number of decays per second. Activity is characteristic of the entire disintegrating substance, not a separate kernel. The practical unit of activity is Curie. 1 Curie is the Isle of the broken kernels contained in the radium for 1 sec platforms / s). The smaller units are used - millikeri and microcures. In the practice of a physical experiment, sometimes another activity unit is used - reprinters / sec.
Statistical character of radioactive decay. Radioactive decay - phenomenon fundamentally statistical. We cannot say when this core is divided, and we can only indicate how likely it disintegrates for one or another time.
Radioactive nuclei is not "aging" in the process of their existence. They generally not applicable concept of age, and you can only talk about the average time of their life.
From the statistical nature of the law of radioactive decay it follows that it is carried out strictly when Veliko, and with small fluctuations should be observed. The number of disintegrating nuclei per unit of time should fluctuate around the average value, characterized by the above law. This is confirmed by the experimental measurements of the number of particles emitted by the radioactive substance per unit of time.

Fig. 30. The dependence of the logarithm of activity of time
Fluctuations are subject to Poisson's law. Producing measurements with radioactive preparations, it is always necessary to take into account this and determine the statistical accuracy of experienced results.
Definition of constant decay X. When determining the constant decay of the X radioactive element, the experience is reduced to the registration of the number of particles flying out of the drug per unit of time, i.e. it is determined by its activity is then built a graph of activity change over time, usually in a semi-lugment scale. The type of dependency obtained in studies of pure isotope, a mixture of isotopes or a radioactive family is different.
Consider as an example several cases.
1. One radioactive element is investigated, with a decay of which stable kernels are formed. Logarithming expression (41), we get
Consequently, in this case, the logarithm of activity is linear function time. The schedule of this dependence has the form of a straight line, the tangent of the angle of inclination (Fig. 30)
2. The radioactive family is investigated, in which there is a whole chain of radioactive transformations. The kernels resulting from decay, in turn themselves turn out to be radioactive:
An example of such a chain can be a decay:

We will find the law describing in this case a change in the number of radioactive atoms in time. For simplicity, we allocate only two elements: counting and the initial, and in the intermediate.
Then the change in the number of nuclei A and nuclei in determined from the system of equations
The number of nuclei and decreases due to their collapse, and the number of cores in decreases due to the decay of the cores in and increases due to the collapse of the core A.
If there are nuclei, but no nuclei, then the initial conditions will be recorded in the form
The solution of equations (43) has the form
and the total activity of the source consisting of nuclei A and in:
We now consider the dependence of the logarithm of radioactivity from time to different ratios between and
1. The first element is short-lived, the second is long-lived, i.e.. In this case, the curve showing the change in the total activity of the source has the appearance presented in Fig. 31, a. At the beginning of the course of the curve is determined mainly by a rapid decrease in the number of active nuclei of the nuclei in also disintegrate, but slowly, and therefore their decay does not significantly affect the slope of the curve on the site. In the future, the nuclei of type A remains in a mixture of isotopes a little, and the slope of the curve is determined by the constant decay if you need to find and then at the inclination of the curve when great meaning The time is found (in terms of (45) the first exponential member in this case can be discarded). To determine the magnitude, it is also necessary to take into account the effect of the decay of the long-lived element on the slope of the first part of the curve. To do this, extrapolate direct to the area of \u200b\u200bsmall times, at several points are subtracted from the total activity, the activity defined by the element in the obtained values
build a straight line for the element A and in the corner they find (while it is necessary to move from logarithms to antilogariffs and back).

Fig. 31. The dependence of the logarithm activity of the mixture of two radioactive substances from time to time: and - when
2. The first element is long-lived, and the second short-lived: dependence in this case has the appearance presented in Fig. 31, b. At the beginning, the activity of the drug increases due to the accumulation of nuclei V. Then comes radioactive equilibrium, in which the ratio of the number of nuclei and to the number of nuclei in becomes constant. This type of equilibrium is called transition. After some time, both substances begin to decrease with the rate of decay of the mother element.
3. The half-life of the first isotope is much more than the second (it should be noted that the half-life of some isotopes is measured by millions of years). In this case, the so-called age-old equilibrium is established in time, in which the number of nuclei of each isotope is proportional to the half-life of this isotope. Ratio
§ 15th. Law of radioactive decay
The appearance of "manual" scintillation counters And mainly, Geiger Muller Counters, which helped automate particle counts (see § 15th), led physicists to an important conclusion. Any radioactive isotope is characterized by spontaneous weakening of radioactivity, expressing in reducing the number of disintegrating nuclei per unit of time.
Construction of graphs of activity of various radioactive isotopes led scientists to the same dependence expressed indicative function (See schedule). According to the horizontal axis, the observation time is postponed, and on the vertical - the number of unspoken nuclei. The curvature of the lines could be different, but the function itself, which was expressed by the dependence described by charts, remained the same:
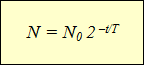
This formula expresses the law of radioactive decay: The number of unprecedented nuclei is defined as the work of the initial number of nuclei by 2 to the degree equal to the ratio of the observation time by the half-life taken with a negative sign.
As it turned out in the course of the experiments, various radioactive substances can be described in various half-life - time, for which the number of unprecedented nuclei decreases twice (See Table).
Periods of half ward some isotopes of some chemical elements. The values \u200b\u200bfor both natural and artificial isotopes are given.
| Iodine-129. | 15 million years | Carbon-14. | 5.7 thousand years | |
| Iodine-131. | 8 days | Uranus-235 | 0.7 billion years | |
| Iodine-135 | 7 o'clock | Uranus-238. | 4.5 billion years old |
The half-life is a generally accepted physical value that characterizes the speed of radioactive decay. Numerous experiments show that Even with a very long observation of the radioactive substance, its half-life is constant, that is, it does not depend on the number of already broken atoms. Therefore, the law of radioactive decay found an application in the method of determining the age of archaeological and geological finds.
The method of radiocarbon analysis. Carbon is a very common chemical element, which consists of stable carbon-12 isotopes, carbon-13 and carbon-14 radioactive isotope, whose half-life is 5.7 thousand years (see table). Living organisms, consuming food, accumulate all three isotop in their fabrics. After the body's cessation of the body is stopped, carbon compensation stops, and over time, its content decreases naturally, due to radioactive decay. Since only carbon-14 decomposes, over the centuries and millennia, the ratio of carbon isotopes in fossil remains of living organisms changes. Measuring this "carbon proportion", one can judge the age of archaeological find.
The method of radiocarbon analysis is also applicable for geological breeds, as well as for fossil objects of human resources, but provided that the ratio of isotopes in the sample was not violated during its existence, for example, by fire or action of a strong radiation source. Injecting such reasons immediately after the opening of this method, it led to errors for several centuries and millennia. Today, "age-old calibration scales" are used for carbon isotop-14, based on its distribution in long-lived trees (for example, in the American millennial sequoy). Their age can be calculated quite accurately - on the annual rings of wood.
The limit for the application of the radio-carbon analysis method in early XXI century amounted to 60,000 years. To measure the age of more ancient samples, such as rock or meteorites, use a similar method, but instead of carbon, uranium isotopes or other elements are observed depending on the origin of the sample under study.
JavaScript is disabled in your browser.The term "radioactivity", called from the Latin words "Radio" - "radiating" and "Activus" - "effective", means the spontaneous transformation of atomic nuclei, accompanied by the emission of gamma radiation, elementary particles or more lung nuclei. Based on all famous science Types of radioactive transformations are fundamental (strong and weak) interactions of particles that are part of the atom. Unknown to this appearance of penetrating radiation emitted by Uranus, in 1896, the French scientist Antooman Henri Beququel, and in a wide use of the concept of "radioactivity" introduced at the beginning of the 20th century Maria Curie, which, exploring invisible rays emitted by some minerals, managed to allocate Pure radioactive element - radium.
Differences of radioactive transformations from chemical reactions
The main feature of radioactive transformations is that they occur spontaneously, while for chemical reactions in any case, any external influences are required. In addition, radioactive transformations occur continuously and are always accompanied by the release of a certain amount of energy, which depends on the interaction force of atomic particles among themselves. Neither the temperature nor the presence of electrical and magnetic fields, nor the use of the most efficient chemical catalyst, nor the pressure nor the aggregate state of the substance, does not affect the rate of flow of reactions inside atoms. Radioactive transformations do not depend on external factor And there can be neither accelerated or slowed down.
Law of radioactive decay
The intensity of the radioactive decay, as well as its dependence on the amount of atoms and time, is expressed in the law of radioactive decay, open by Ernest Rutherford and Frederic Soddy in 1903. In order to come to certain conclusions, subsequently reflected in the new law, scientists conducted the following experiment: they separated one of the radioactive products and studied its independent activity separately from the radioactivity of the substance from which it was highlighted. As a result, it was found that the activity of any radioactive products regardless of chemical element Over time, decreases in geometric progression. Based on this, scientists concluded that the speed of radioactive transformation is always proportional to the number of systems that have not yet been converted.
The formula of the law of radioactive decay is as follows:
according to which the number of decays -dn, which occurred during the period of time DT (very short interval), in proportion to the number of atoms N. In the formula of the Radioactive decay, there is another important amount - a constant decay (or the inverse amount of the half-life) λ, which characterizes the probability of the core decay per unit time.
What chemical elements are radioactive?
The instability of atoms of chemical elements is rather an exception than regularity; For the most part, they are stable and over time do not change. However, there is a certain group of chemical elements whose atoms are more than others are decomposed and, decaying, emitting energy, and also allocate new particles. The most common chemical elements are radium, uranium and plutonium, which have the ability to turn into other elements with simpler atoms (for example, uranium turns into lead).
The phenomenon of radioactivity was open in 1896 by A. Becquerem, who observed the spontaneous emission of the salts of uranium unknown radiation. Soon E. Rutherford and spouses Curie found that the nuclei (α-particles), electrons (β-particles) and tough are emitted during radioactive decay. electromagnetic radiation (γ-rays).
In 1934, the decay with the departure of positrons (β + -Respad) was opened, and in 1940 it was opened new Type Radioactivity - Spontaneous division of the nuclei: The core divided into two fragments of comparable mass with the simultaneous emitting of neutrons and γ -Kvanta. The proton radioactivity of the nuclei was observed in 1982. Thus, there are the following types of radioactive decay: α-decay; -Spad; - disintegration; E - Capture.
Radioactivity- the ability of some atomic nuclei spontaneously (spontaneously) to turn into other kernels with the emission of particles.
Atomic nuclei consist of protons and neutronswho have a generalizing name - nucleons. The number of protons in the kernel determines chemical properties Atom and is designated Z. (serial number element). Number of nucleons in the kernel are called mass number And denote BUT. Kernel with the same sequence number and various mass numbers are called isotopes. All isotopes of one chemical element have the same characteristic properties, and physical properties May differ very strongly. To refer to isotopes, a symbol of a chemical element with two indices is used: A Z H.. Lower index - sequence number, upper - mass number. Often the lower index is lowered, as the element symbol itself indicates on it.
For example, they write 14 with instead of 14 6 C.
The ability of the kernel to decay depends on its composition. The same element may also have stable, and radioactive isotopes.
For example, carbon isotope 12 s is stable, and isotope 14 with radioactive.
Radioactive decay is a statistical phenomenon. The ability of the isotope to decay characterizes the constant decay λ.
The constant decay is the likelihood that the core of this isotope will be dispersed per unit of time.
Denote the number N of the radioactive decay cores at the time of T, DN 1 - the number of nuclei of the broken during DT. Since the number of nuclei in the substance is enormous, the law of large numbers is performed. The probability of the decomposition of the core over a small time DT is by the formula dp \u003d λdt. Trust is equal to the probability: d n 1 / n \u003d dp \u003d λdt. d n 1 / n \u003d λdt - Formula Determining the amount of nuclei who have broken.
The solution of the equation is: - the formula is called the law of radioactive decay: The number of radioactive cores decreases with time according to the exponential law.
Here, n - the number of nuclei of the nuclei by time T; N O is the initial number of unreacted nuclei; λ is a constant radioactive decay.
In practice, not constant decay λ , and the magnitude called half-life T..
The half-life (T) period is the time during which half-phaseactive nuclei disintegrates.
Radioactive decay law half-life (T) has the form:
![]()
The relationship between the half-life period and the constant decay is determined by the formula: t \u003d ln (2 / λ) \u003d 0.69 / λ
The half-life can be both very large and very small.
To assess the degree of activity of radioactive isotope, a value called activity is used.
Activity number of radioactive drug nuclei disintegrated per unit of time: a \u003d dn weld / dt
For a unit of activity in Si, 1 Beckel (BC) \u003d 1 decay / C is the activity of the drug in which 1 ° C occurs 1 decay. Larger activity unit - 1 Rangeford (RD) \u003d BC. A generic unit of activity - Curie (CI) is often used, equal to the activity of 1 g radium: 1 ki \u003d 3.7 BK.
Over time, activity decreases by the same exponential law, which disintegrates the radionuclide itself:
![]() =
.
=
.
In practice, the formula is used for calculatation:
A \u003d. \u003d λn \u003d 0,693 n / t.
If we express the number of atoms through the mass and painting mass, then the formula for calculativity will take the form: a \u003d \u003d 0.693 (μt)
where is the number of Avogadro; μ - molar mass.