INSTRUCTIONS AND PROPHECIES OF THE Blessed MOTHER ALIPIA GOLOSEEVSKY, Kyiv...


You know that particles in bodies are in constant random motion. Why doesn't a solid body break up into separate particles? This is because the particles (molecules or atoms) of most solids are arranged in a certain order and very close to each other.
Each particle attracts neighboring particles to itself and is itself attracted to them. These forces hold, for example, iron atoms in a piece of metal, water molecules in a piece of ice or in a drop of water. In other words, attractive force is the force that holds particles together.
If you break the knitting needle into two parts and put them together, then they will not be held together. It turns out that attraction between the particles of matter becomes possible only when they are at a certain distance, close enough to each other.
Experience makes it possible to detect the attraction of particles.
They take a small lead cylinder, cut it into two halves and quickly move them with fresh cuts. If the cut did not have time to oxidize, then both parts of the lead cylinder will join into one. This can be checked by fixing one of the cylinders in the holder, and hanging a load from the other. Half of the cylinder with the load does not fall. Consequently, the molecules of the halves of the cylinder interact with each other.
Rice. 34. Attraction of particles. The two halves of the lead cylinder are connected by the interaction of molecules.
The described experiment succeeds due to the softness of lead. With bodies harder than lead (for example, halves broken glass) it is impossible to carry out such an experiment.
For a bond to occur, the molecules must be at a distance of several smaller sizes the molecules themselves. Pieces of soft material, such as plasticine, stick together easily. This is because they can be brought close to a distance at which the forces of attraction act.
The structure of liquids is different from the structure of solids. In liquids, the interaction between molecules is weaker than in solids, but it still exists. Imagine that water is poured into a glass and then poured into a flask. Initially, the liquid took the form of a glass, and then a flask into which it was poured. If in water there was an attraction between the molecules of the same force as in solids, then its shape could not change so easily.
Molecules in liquids are located almost close to each other, so all liquids have very little compressibility. But the interaction between molecules is not strong enough for liquids to retain their shape. This explains the main property of liquids - fluidity.
We have already said that gas can be compressed so that its volume decreases several times. This means that in gases, the distance between molecules is much greater than the size of the molecules themselves. In such cases, the molecules are weakly attracted to each other. This is why gases do not retain their shape and volume.
There is mutual attraction between particles in solids, liquids and gases.
The question arises: “Why are there gaps between particles?” It would seem that the particles, being attracted to each other, should “stick together”. The compression of bodies, however, is prevented particle repulsion. That this is so can be seen from an example. A rubber eraser that is compressed and folded in half will straighten out when the edges are released. Compressed bodies straighten out because, when compressed, the particles are so close together that they begin to repel each other. Consequently, attraction between particles – atoms and molecules, keeps them close to each other, and repulsion prevents their complete convergence.
Why do many solids have great strength? On the steel cable with a thickness of only 25 mm, a diesel locomotive can be lifted. It is difficult to divide the stone into pieces. This can be explained by the attraction of the particles that make up solids. Molecules (atoms) in solids are attracted to each other. But why then the pieces of a broken glass cup cannot be joined together without glue into one whole? At the same time, pieces of plasticine can be easily joined into one piece. Do this experience yourself.
These facts can be explained by assuming that the attraction of molecules (atoms) is manifested only at small distances between them. Indeed, if you heat the glass pieces so that the glass becomes soft, and press them against each other, they stick together into one whole.
Molecules of the liquid are also attracted. Let's do an experiment. We hang a clean glass plate on the spring and mark the position of the lower end of the spring with a pointer (Fig. 106, a). We bring a vessel with water to the plate until it comes into contact with the surface of the water (Fig. 106, b), after which we lower the vessel until the plate comes off. The extension of the spring will increase, which indicates the attraction of the particles of the liquid (water) in the vessel and on the surface of the glass plate.
Rice. 106
But the molecules (atoms) of the gas are practically not attracted to each other. In gases, particles are located at greater distances than in liquids and solids. The attraction at these distances is negligible. Therefore, the gas molecules scatter over the entire volume provided by the gas. For example, the smell of perfume from an open bottle spreads throughout the room.
Is there repulsion between molecules?
Take a solid rubber ball and try to compress it (Fig. 107, a). Is it easy to do? One has only to stop squeezing the ball, as it immediately restores its shape (Fig. 107, b). Means, between particles ball there is a repulsion. It was the repulsion of particles that made it difficult to compress the ball, it also restored its original shape.

Rice. 107
It is very important to understand that the attraction and repulsion of particles of matter manifests itself only at small distances between particles, i.e., in solids and liquids, and changes noticeably with these distances. Describing the interaction of molecules, we will model them with balls. So, at certain distances, the attraction of two molecules is compensated (balanced) by repulsion (Fig. 108, a). When the molecules move away (Fig. 108, b), the repulsion becomes less than the attraction, and when the molecules approach (Fig. 108, c), the repulsion becomes greater than the attraction.

Rice. 108
The interaction of two molecules in the body can be conditionally compared with the interaction of two balls held together by a spring (Fig. 109, a). At distances r\u003e r 0 (the spring is stretched), the balls are attracted to each other (Fig. 109, b), and at distances r< r 0 (пружина сжата) - отталкиваются (рис. 109, в).

Rice. 109
Although this model is illustrative, it has a drawback: it shows either attraction or repulsion between the balls. Between the particles of matter, attraction and repulsion exist simultaneously! At some distances (when the particles move away), attraction prevails, and at others (when they approach each other), repulsion prevails.
Think and answer
Do it yourself at home
Interesting to know!
If you carefully clean the ends of two lead cylinders with a knife or blade and press them tightly against each other, then the cylinders “stick together”. The mutual attraction of the cylinders is so great that they can hold a weight of mass m = 5 kg (Fig. 110).

Rice. 110
"Adhesion" of lead cylinders proves that particles of substances are able to attract each other. However, this attraction occurs only when the surfaces of the bodies are very smooth (for this, cleaning with a blade was needed). In addition, the bodies must be tightly pressed against each other so that the distance between the surfaces of the bodies is comparable to the distance between the molecules.
§ 07-g. Interaction of particles of substances
In the two previous paragraphs, we discussed experiments illustrating the first and second propositions of the MKT. Let us now consider experiments illustrating the third basic proposition of the MKT and its consequences.
For the experiment, we take two lead cylinders with hooks. To remove dust particles, we clean the ends of both cylinders to a shine with a knife or blade (Fig. a). By firmly pressing the ends to each other, we will find that the cylinders are firmly “clutched”. The strength of their adhesion is so great that, if the experiment is carried out successfully, the cylinders can withstand the weight of a weight of up to 5 kg (Fig. b). From this experience follows the conclusion: particles of matter are attracted to each other. However, this attraction is noticeable only when the surfaces of the bodies are very smooth and, moreover, tightly adjoin each other.
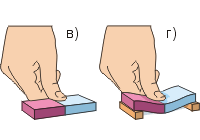
Let's do the second experiment (Fig. c, d). It takes a lot of pressure to squeeze the rubber eraser with your finger. great strength; The eraser is easier to bend than to squeeze. Other bodies (except gaseous) are also very difficult to squeeze. This suggests that particles of matter repel each other.
Attraction and repulsion of particles of substances arise only if the particles are in close proximity to each other. Usually, at distances, large sizes the particles themselves, they are attracted; at distances smaller than the particle size, they repel each other. If the particles are removed at a distance many times greater than their size, the interaction almost does not appear.
Let us now consider the energy aspect of particle interaction.

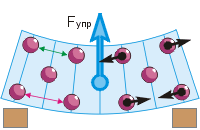
If any bodies interact, they have potential energy depending on the mutual position of these bodies (see § 5-e). In the figure on the right, the arrows on the particles show the repulsive forces of the "neighbors". The same could be said about the forces of attraction. If all particles were at equal distances from each other, then all forces would be mutually balanced ("green" particle). However, according to the second position of the MKT, the particles move. Therefore, the distances from each particle to its neighbors change all the time (the “red” particle). Consequently, the forces of their interaction are constantly changing and not balanced. With these changes in distances and forces the potential energy of each particle changes, taking the minimum value in the position of its equilibrium.
The potential energy of a particle is considered to be zero when it is at a great distance from other particles, as, for example, in gases, where there is practically no interaction between particles (see Fig. § 7-b). in hard and liquid substances there is an interaction of particles, which means that there is also a potential energy of particles (we note in parentheses: it is negative, but now its value in absolute value is important for us). And in order to overcome the interaction of particles and separate them at a distance, you need to do work. And, the more work to overcome the interaction of particles to separate them at a distance, the greater (in modulus) the potential energy of interaction of particles of the substance under study.
The emergence of elastic force. Compressing or stretching, bending or twisting the body, we bring together or remove its particles (see figure). That's why the forces of attraction and repulsion of particles change, the joint action of which manifests itself as an elastic force.
Let's return to the bend of the eraser (fig. d). We conditionally depicted rubber particles as balls. When pressed with a finger, the upper particles approach each other (“green” distance is less than “red”). This leads to the emergence between them repulsive forces(black arrows point away from the particles). The lower particles move away from each other, which leads to the appearance between them attraction forces(black arrows are directed towards the particles). As a result, the eraser tends to straighten up, which means that there is an upward elastic force in it - opposite to the finger pressure force.
Javascript is disabled in your browser.The interaction of particles with matter depends on their type, charge, mass and energy. Charged particles ionize the atoms of matter by interacting with atomic electrons. Neutrons and gamma quanta, colliding with particles in matter, transfer their energy to them, causing ionization as a result of the formation of secondary charged particles. In the case of γ-quanta, the main processes leading to the formation of charged particles are the photoelectric effect, the Compton effect, and the creation of electron-positron pairs. The interaction of particles with a substance depends on such characteristics of the substance as its density, atomic number and average ionization potential of the substance.
|
Rice. clause 4.1. Interaction of a particle with matter. |
A heavy nonrelativistic charged particle with charge Ze and velocity v flies along the x axis at a distance ρ from the electron (Fig. 2.2). The force of interaction at the moment of closest approach of the particles F = Ze 2 / ρ 2 . Interaction time Δt ≈ 2 ρ /v . The momentum transferred to the electron is Δp ≈ FΔt = 2Ze 2 / (ρ v) . Transferred energy
ΔE ≈ (Δp) 2 /2m e = 2Z 2 e 4 /(m e v 2 ρ 2). If n is the number of electrons in a volume unit, then the number of electrons in a volume element
∆N = 2πρndρdx. The total energy transferred to the electrons,
where m e is the electron mass (m e c 2 = 511 keV is the rest energy of the electron); c is the speed of light; β = v/c; v is the speed of the particle; Z is the particle charge in units of the positron charge; n e is the electron density of the substance; is the average ionization potential of the atoms of the substance of the medium through which the particle passes:
= 13.5Z "
eV, where Z "
is the charge of the nuclei of the substance of the medium in units of the charge of the positron;
r 0 \u003d e 2 / (m e c 2) \u003d 2.818 10 -13 cm - the classical radius of the electron.

Rice. p4.2. Specific energy loss of a charged particle in air.
The passage of electrons through matter differs from the passage of heavy charged particles. main reason- a small mass of the electron, which leads to a relatively large change in the momentum of the electron with each collision with particles of matter, causing a noticeable change in the direction of the electron and, as a result, electromagnetic radiation.
The specific energy loss of electrons with kinetic energy Te is the sum of ionization and radiation energy losses.
| (clause 4.3) |
In the region of low electron energies (T e< 1 МэВ) определяющий вклад в потери энергии дают неупругие ионизационные процессы взаимодействия с атомными электронами, включающие ионизацию атомов. Передаваемая в одном столкновении энергия в среднем мала и при движении в веществе потери складываются из очень большого числа таких малых потерь энергии.
Ionization energy losses of electrons dominate in the region of relatively low energies. As the electron energy T e increases, the radiative energy loss increases. According to classical electrodynamics, a charge experiencing acceleration a radiates energy. The radiation power W is determined by the relation W = (2/3)e 2 a 2 /c 3 . Acceleration of a particle with charge z in the field of an atomic nucleus with charge Z: a ≈ Zze 2 /(mr 2).
The acceleration is inversely proportional to the particle mass m. Therefore, the energy emitted during deceleration of a proton is less than the energy emitted by an electron in the same field by ~3.5·10 6 times. Radiation losses, which play an important role in the deceleration of high-energy electrons, are practically insignificant when heavy charged particles pass through matter.
 |
E<< m e с 2 = 511 кэВ, |
 |
|
 |
The ratio between radiation and ionization specific energy losses of electrons for a liquid and a solid is determined by the relation:
|
|
(clause 4.4) |
The energy at which the energy losses for radiation and ionization become the same is called critical.
Heavy charged particles interact mainly with atomic electrons and therefore deviate little from the direction of their initial motion and move almost in a straight line. The average length of the path traversed by the particle to complete deceleration coincides with the distance from the entry point of the particles into the substance to the point of their stop and is called the particle path. Typically, mileage is measured in units of length (m, cm, microns) or length times the density of the substance (g / cm 2).
The range of α-particles in various substances depending on the energy T α
| T α , MeV | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Air, cm | 2.5 | 3.5 | 4.6 | 5.9 | 7.4 | 8.9 | 10.6 |
| Al, µm | 16 | 23 | 30 | 38 | 48 | 58 | 69 |
| Biological tissue, microns | 31 | 43 | 56 | 72 | 91 | 110 | 130 |
The range of protons in aluminum depending on the energy T p
In the energy range of γ-quanta from 10 KeV to 10 MeV, three mechanisms of interaction of γ-quanta with matter are most significant:
photoelectric effect– the process of interaction of g-quanta with the electron of the atomic shell. An electron flies out of an atom with a kinetic energy T e = E γ – I i , where E γ is the energy of the γ-quantum, I i is the ionization potential of the i-th electron shell of the atom. Compton effect
– the process of photon scattering by a free electron, in which the wavelength of the scattered photon changes. Formation of electron-positron pairs
occurs in the field of the atomic nucleus at the energy of the γ-quantum E γ ≥ 2m e c 2 or on the electron at E γ ≥ 4m e c 2 .
As a result of interactions in matter, the intensity of the γ-ray beam is weakened. The attenuation of the intensity of a monoenergetic beam of γ-quanta is described by the relation
Here N is the number of medium nuclei in 1 cm 3 .

Rice. p4.3. Dependence of the linear absorption coefficient in aluminum and lead on the energy of γ-quanta
The absorption coefficient μ depends on the energy of γ-quanta and the properties of the substance. Exact relationships for the cross sections of the photoelectric effect, the Compton effect, and the pair formation effect can be obtained by quantum electrodynamics methods. The following relations are used to estimate the cross section values:
where r e = e 2 /(m e c 2), ε = E γ /(m e c 2).
| For ε<< 1: |
|
(clause 4.10) |
| For ε >> 1: |
|
(clause 4.11) |
| For m e c 2<< E γ << 137m e c 2 Z -1/3 |
 |
(clause 4.12) |
| At E γ >> 137m e c 2 Z -1/3 |
|
(clause 4.13) |
Cherenkov radiation is a coherent radiation of dipoles formed as a result of the polarization of the medium by a passing charged particle, and occurs when these dipoles (polarized atoms) return to their original unpolarized state. If the particle moves slowly, then the dipoles have time to turn in its direction. In this case, the polarization of the medium is symmetric with respect to the coordinate of the particle. The radiation of individual dipoles cancel each other out when returning to the initial state. When a particle moves with a "superluminal" speed for a given medium, due to the delayed reaction of the dipoles, they are predominantly oriented in the direction of the particle's motion. The resulting polarization turns out to be asymmetric with respect to the location of the particle, and the radiation of the dipoles is uncompensated.
The wave front of Cherenkov radiation (Fig. 2.5) is the envelope of spherical waves emitted by the particle. Photons are emitted at an angle θ to the direction of particle motion:
cosθ = (βn) -1 ,
where β = v/c, n is the refractive index of the medium. The envelope of light waves A for a particle moving with a speed v > c/n is a cone with an opening angle of 2φ, the apex of which coincides with the position of the particle at a given moment (point P " in the figure), and the normals to the generatrices of the cone show the direction of propagation of the Cherenkov radiation.
P 4.1. How many times do the energy losses of protons and K + -mesons with kinetic energy T = 100 MeV differ in aluminum foil 1 mm thick?
P 4.2. A proton beam with a kinetic energy T = 500 MeV and a current I = 1 mA passes through a copper plate with a thickness D = 1 cm. Calculate the power W dissipated by the beam in the plate.
P 4.3. Determine the critical electron energies for carbon, aluminum and iron.
P 4.4. It is necessary to absorb an electron with an energy of 2 MeV in an aluminum absorber. Determine its thickness.
Answer: D = 0.35 cm
P 4.5. What energy does an electron with an energy of 500 MeV lose when passing through an aluminum absorber 1 cm thick?
P 4.6. A radioactive source emits a γ-quantum with an energy of 1 MeV. What should be the wall thickness of the lead container in order to attenuate the radiation intensity 1) by a factor of 103, 2) by a factor of 105?
P 4.7. How do energy transfers of heavy and light charged particles to matter take place?
P 4.8. How do the specific ionization losses of particles depend on the characteristics of the medium in which they move?
P 4.9. Calculate the ratio of specific ionization energy loss of 10 MeV α-particles in air, carbon, and lead.
P 4.10. Calculate the specific ionization energy loss of protons with energies of 1 MeV, 10 MeV, 100 MeV and 1 GeV in lead.
P 4.11. A proton with a kinetic energy of 10 MeV collides with an electron at rest. Calculate the maximum energy the electron will receive.
P 4.12. Calculate what kinetic energy T will be acquired by an initially resting electron when passing by it with an impact parameter ρ of a particle with mass M and charge Z. Particle speed before collision v<< c.
Answer:

P 4.13. Electrons and protons with an energy of 50 MeV fall on an aluminum plate 2 mm thick. Determine the energies of electrons and protons at the output of the plate.
Answer: T p =40.7 MeV, T e =46.4 MeV
P 4.14. Calculate the critical electron energies for air, water, and lead.
P 4.15. Calculate the specific radiation and ionization energy losses of an electron with an energy of 100 MeV when passing through aluminum and lead foil.
Answer: Al: (dT e /dx) ion = 6.2 MeV/cm, (dT e /dx) rad = 10.1 MeV/cm;
Pb:(dT e /dx) ion = 4.3 MeV/cm, (dT e /dx) rad = 44 MeV/cm
P 4.16. Calculate the cross sections for the photoelectric effect, Compton scattering, and production of e + e - pairs when Al is irradiated with γ-quanta with energies 1) 1 MeV, 2) 5 MeV, 3) 50 MeV.
P 4.17. Calculate the cross sections for the photoelectric effect, Compton scattering, and production of e + e – pairs during irradiation with γ-quanta with an energy of 5 MeV of targets made of carbon, iron, and lead
P 4.18. How does the charge of a substance Z on the relative contribution of the cross sections of the photoelectric effect, Compton scattering and the production of e + e - pairs to the total cross section of the interaction of γ-quanta with matter for photons with energies 1) 1 MeV, 2) 5 MeV, 3) 10 MeV and 4) 100 MeV?
particle atom elementary quark
The most important question of physics is the question of interactions. If it were not for the interactions, then the particles of matter would move independently, unaware of the existence of other particles. Thanks to interactions, particles acquire, as it were, the ability to recognize other particles and respond to them, due to which collective behavior is born. Because all matter is made up of particles, to explain the nature of forces, it is necessary, ultimately, to turn to elementary particle physics. By doing this, physicists discovered that all interactions, no matter how they manifest themselves on a large scale, can be reduced to four fundamental types: gravitational, electromagnetic, and two types of nuclear.
Dominated at the quark level nuclear interactions. Strong interaction connects quarks into protons and neutrons and prevents the nuclei from falling apart. dominated at the atomic level electromagnetic interaction linking atoms and molecules. On an astronomical scale, it becomes dominant gravitational interaction.
In recent years, physicists have become interested in the relationship between the four fundamental forces that collectively govern the universe. Is there any connection between them? Are they not just different hypostases of the one fundamental superpowers? If such a superpower exists, then it is precisely this that represents the active principle of all activity in the Universe - from the birth of subatomic particles to the collapse of stars. Unraveling the mystery of superpower would increase our power over nature unimaginably and even allow us to explain the very "creation" of the world.
We already know that elementary particles interact with each other through other particles, which they continuously emit and absorb. The layers of these particles shield the charges, so a particle from different heights to it looks charged in different ways. That is how, always differently charged, colliding particles see each other. The greater their energy, the deeper they penetrate each other and the more clearly they feel the “breathing” of their central unshielded charges. Therefore, it can be expected that with increasing energy, different types of interactions will become more and more similar and at high energies merge into one single interaction - superforce. There will be a "great unification" of all the forces of nature.
The real state of affairs is somewhat more complicated. Screening clouds are formed not only around the charge, but also around each carrier particle, with which colliding particles probe each other. If the interaction carriers are very heavy, then the interaction is transferred to ultrasmall distances. Far from the center, such particles are almost never encountered, and the interaction associated with them manifests itself very weakly. In other cases, carriers are light (for example, photons), they are able to travel far from the charge that emitted them, and with their help, interaction occurs at large distances.
Thus, not only particles, but also the forces that bind them, turn out to be extraordinarily complex. You can’t call them the simplest points! And it's hard to believe that the gravitational force of two electrons and the billions greater force of their electromagnetic repulsion are branches of the same tree.
Physicists came to the idea of a "great unification" quite recently - some twenty or thirty years ago, although the first step was taken by Faraday and Maxwell, who combined electricity and magnetism, which, as it was then believed, were completely different interactions. They also introduced the concept of "field". Faraday proved that electricity and magnetism are two components of the same electromagnetic field.
The next step towards the "great unification" was much more difficult. It was made only in the mid-60s of the twentieth century. The attention of physicists was then attracted by the weak interaction. It had a strange feature: for all other forces, an intermediate field can be indicated, the quanta of which serve as a carrier of interaction, and in decay processes, the particles "talk" so to speak, directly, without any intermediaries, pushing each other like billiard balls.
It is natural to assume that in this case there is also an exchange between particles, but only so heavy that the whole process occurs at very small distances, and from the outside it looks as if the particles are simply pushing each other.
Calculations showed that if it were not for the large mass of intermediate particles, then such an interaction in its properties would be very similar to electromagnetic. And here are three physicists: Abdus Salam, Steve Weinberg and Sheldon Gleshow admitted that the photon and heavy intermediate particles of weak interaction are the same particle, only in different "fur coats". The theory developed by them began to be called "electroweak", since, as a special case, it contains electrodynamics and the old theory of weak interactions. Soon, heavy quanta of the electroweak field were caught on accelerators - three meson brothers with a mass almost a hundred times more than a proton. The creation of the theory of the electroweak field and the experimental discovery of its carriers were awarded two Nobel Prizes at once.
Inspired by the discovery of the electroweak field, physicists were carried away by a new idea for further unification - the merging of the strong interaction with the electroweak one. The essence of this idea is as follows. Each quark has an analogue of electrical charge, called a color. Unlike charge, there are three kinds of colors in a quark. Therefore, the gluon field is more complex. It consists of eight component force fields. In a typical hadron - a proton or a neutron - the combination of three quarks - red, green and blue - always has a "white" color. The emitted mesons contain quark-antiquark pairs, so they are also "colorless". Since we know that when particles interact, their charges are screened, this leads to those effects of differences in the range of interactions of different types of particles. An estimate of the distance at which all interactions become comparable in magnitude is about 10 to -29 centimeters. The interaction carrier - the X-particle - has a mass equal to about 10 to the 14th power of the proton mass. During that insignificant period of time that the X-particle exists, energy and mass have a huge uncertainty. And in this respect we are like Thales and other Greek philosophers who speculated about the properties of atoms without the slightest hope of ever seeing them.
Elementary particles cannot be divided into simpler parts (which is why they were called "elementary"). In any reactions known today, these particles only pass into each other - they interconvert. Moreover, heavier particles can be born from the lungs - if they move at a sufficient speed (kinematic energy goes into mass)
Elementary particles differ in charge, spin, mass, lifetime, and so on. For example, the lifetime of a proton is longer than the lifetime of the Universe, and the rho-meson lives 10 to -23 degrees of a second. The mass of photons and neutrinos is equal to zero, and the mass of the maximon (the heaviest elementary particle that can only exist), which has not yet been discovered, but predicted by theorists, is something about a microgram - like a large speck of dust visible to the eye. They can be divided into families, and the members of each can be considered as different states of the same particle. Families are combined into more complex groups - clans, or multiplets. But the main thing is that the multiplets are connected by certain symmetry rules. In general, it turns out something like a periodic table of elementary particles, like Mendeleev's. It can be assumed that physicists have groped for the next tier of the structure of matter.
Accelerators of elementary particles have played an important role in the development of knowledge. Electron transmission showed that the proton is not really a point, but rather a large object with a radius of about 10 to -13 centimeters. Analyzing the results of new experiments on electron scattering, scientists concluded that nucleons are a swarm of some very small particles, which, at a lower magnification, look like a bunch of mesons and other elementary particles overlapping and penetrating each other. Theorists involved in the classification of particles were delighted, since they had long suspected the existence of such particles, they only called them in their own way: quarks.
When quarks appeared on the pages of theoretical articles, many scientists considered them to be just some kind of curiosity, temporary scaffolding on the way to a more perfect theory. However, physicists did not have time to look back, as it turned out that with the help of quarks, a variety of experimental facts are very simply and clearly explained, and theoretical calculations are greatly simplified. It became simply impossible to do without quarks, as well as without molecules and atoms.
Nucleon probing experiments proved that quarks in the center of an elementary particle are almost not connected by interaction and behave like balloons floating in the air. If they try to disperse, then immediately there are forces that pull them together. At the periphery, quarks can only be in the form of bound bunches - for example, in the form of pi-mesons, which is consistent with the theory of nuclear interaction based on mesons. But how do quarks interact with each other? Since science does not know any other way to organize the interaction than by transferring the particle-carrier of the interaction, gluons were proposed - particles gluing quarks together. Gluons are like photons, only with a charge. The photon does not create any field around itself, therefore the field has the greatest intensity near its source - the charge, then it gradually dissipates and weakens. The gluon, on the other hand, gives rise to new gluons with its charge, which, in turn, give rise to the next ones, and so on, so the gluon field does not weaken, but, on the contrary, increases with distance from the quark that gave rise to it. The receding quark, like foam, is overgrown with new gluons and their bond becomes stronger.
Particle physics is an amazing fusion of experiment and theory. The properties of the smallest particles of matter have been established and continue to be established in experiments that are unparalleled in complexity in other areas of science. These unique experiments combine a truly industrial scale with a jeweler's precision. In most cases, the objects of study themselves - particles - are created right there in the laboratory with the help of accelerators and live for such insignificant periods of time that, compared with them, a moment seems like an eternity. The case of some rare decay of a particle has to be found among billions of "uninteresting" decays similar to it. All information about elementary particles is obtained as a result of careful measurements.